
The term peptide bond is used only when both groups come from amino acids. peptide bond: another name for amide bond, a chemical bond formed when a carboxylic acid condenses with an amino group with the expulsion of a water molecule. Molecules or functional groups having a dipole moment are said to be polar. The nonpolar side chains are pushed to the interior of the protein allowing them to avoid water molecule and giving the protein a globular shape.
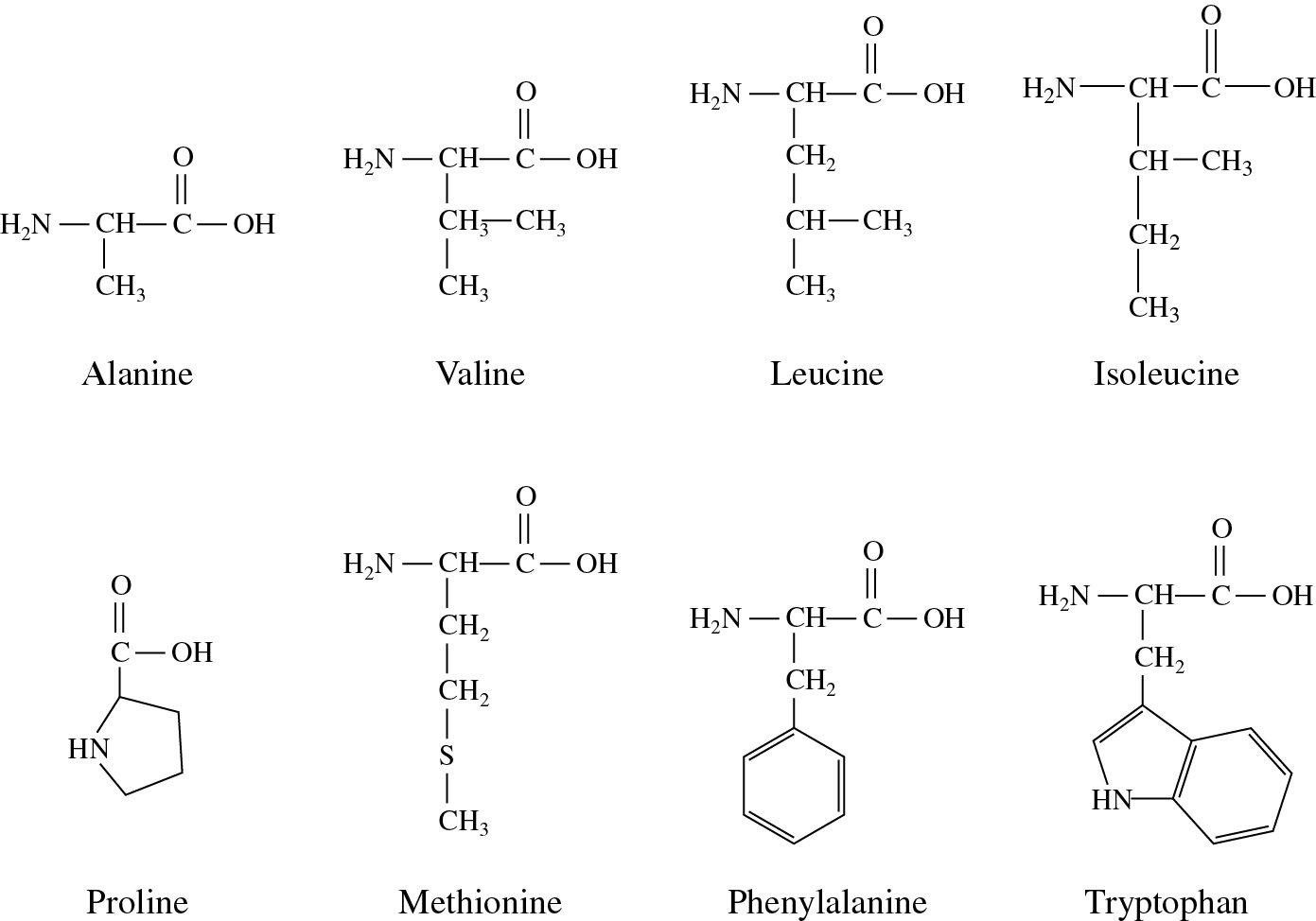
Since proteins have nonpolar side chains their reaction in a watery environment is similar to that of oil in water. With an alpha-amino group at one end and an alpha-carboxyl group at the other, a polypeptide chain has polarity because its ends are distinct. It occurs when the carboxylic group of one molecule reacts with the amino group of the other molecule, linking the two molecules and releasing a water molecule.Ī polypeptide chain contains a sequence of amino acids joined by peptide bonds, and each amino acid unit in a polypeptide is called a residue. The bond that holds together the two amino acids is a peptide bond, or a covalent chemical bond between two compounds (in this case, two amino acids). and -NH groups of the peptide bond are polar ( both hydrophobic and hydrophillic nature) and involved in H-bonding. Hence the peptide bond is a nonpolar covalent bond because it holds together two amino acids. Polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the sharing is unequal.


 0 kommentar(er)
0 kommentar(er)
